Malaria is present in over 100  countries worldwide, and it is estimated that around 3.4 billion people – half of the world’s population – are at risk of infection. There were an estimated 627,000 deaths caused by malaria infection in 2012, with over 90% of deaths occurring in African children.
countries worldwide, and it is estimated that around 3.4 billion people – half of the world’s population – are at risk of infection. There were an estimated 627,000 deaths caused by malaria infection in 2012, with over 90% of deaths occurring in African children.
The disease is caused by Plasmodium parasites including P. falciparum and P. vivax, which are carried by infected Anopheles mosquitoes. Antimalarial drugs can be used to prevent and treat malaria, but resistance to these agents frequently develops. Recent research found that P. falciparum parasites are becoming increasingly resistant to artemisinin therapies in Southeast Asia, highlighting that radical action is required to prevent the spread of malaria and develop new effective therapies.
Vaccination is one of the most promising strategies to prevent malaria infection. While there is no currently licensed vaccine, it is thought that the partially efficacious RTS,S vaccine could soon be approved for use against P. falciparum malaria, as discussed in our previous blog post. A number of vaccines currently under investigation were outlined at the recent Beating Malaria conference, where Irene Gramaglia described candidates for vaccines targeting the pre-erythrocytic, erythrocytic and transmission stages of malaria.
Markers of vivax malaria immunity
In a systematic review and meta-analysis published in BMC Medicine as part of our Combating malaria: research, prevention and treatment article collection, Freya Fowkes and colleagues from the Burnet Institute examined studies investigating antibody expression patterns of people living in P. vivax endemic areas. The authors reveal several specific antibodies associated with infection risk or protective immunity. As the majority of studies included in the meta-analysis were carried out in Brazil, the authors highlight that more studies representing diverse geographical areas are required. However, these findings provide important insights into the mechanisms of protective immunity against P. vivax malaria; the identified antibodies could be used as biomarkers for active infection or protective immunity, and could lead to the design of an effective vaccine. Looking to the future, the authors conclude that:
“Population-based immuno-epidemiology studies are pivotal to identify P. vivax antigens associated with protective immunity and exposure…future studies should aim to represent diverse populations and special consideration should be given to studies in populations that contain considerable migrant sub-populations”
Protection against falciparum malaria
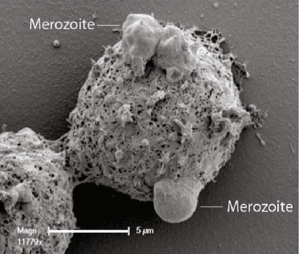 There have also been some novel insights into the mechanisms of P. falciparum infection and protective immunity. In a collaborative study between Australian authors and the KEMRI project, James Beeson and colleagues investigated the mechanisms of protective immunity to P. falciparum malaria in two Kenyan cohorts. The research, published recently in BMC Medicine, reveals that opsonic phagocytosis of P. falciparum merozoites – the mechanism whereby white blood cells engulf the malaria parasites – is associated with reduced risk of clinical malaria in infected individuals. Commenting on this research, Ann Moorman and Ann Stewart explain that antibodies linked to the process of opsonic phagocytosis could be measured as a biomarker of malaria immunity, and could be used in the design of an effective vaccine. Moorman and Stewart highlight that:
There have also been some novel insights into the mechanisms of P. falciparum infection and protective immunity. In a collaborative study between Australian authors and the KEMRI project, James Beeson and colleagues investigated the mechanisms of protective immunity to P. falciparum malaria in two Kenyan cohorts. The research, published recently in BMC Medicine, reveals that opsonic phagocytosis of P. falciparum merozoites – the mechanism whereby white blood cells engulf the malaria parasites – is associated with reduced risk of clinical malaria in infected individuals. Commenting on this research, Ann Moorman and Ann Stewart explain that antibodies linked to the process of opsonic phagocytosis could be measured as a biomarker of malaria immunity, and could be used in the design of an effective vaccine. Moorman and Stewart highlight that:
“In the headlong race to develop potential malaria vaccine candidates, the as yet undiscovered ‘golden chalice’ remains an in vitro assay that can, if not predict the efficacy of a vaccine, at least correlate well with it”
Why is RTS,S only partially efficacious?
As well as elucidating novel mechanisms of malaria immunity in the race to develop an effective vaccine, it is also important to understand why the RTS,S vaccine was only partially efficacious in clinical trials. To address this question, Azra Ghani and colleagues analyzed predictors of vaccine immunogenicity using data from phase II clinical trial participants. RTS,S-induced anti-circumsporozoite protein (CSP) antibody titres were found to be significantly associated with malaria protection, and vary with age, adjuvant use and other antibody levels, suggesting that differences in anti-CSP levels could explain variations in RTS,S vaccine efficacy. Understanding these mechanisms should allow the selection of people most likely to benefit from RTS,S vaccination, and these findings suggest that measuring anti-CSP levels could be an effective method of stratification.
Taken together, the results of these studies reveal new mechanisms mediating protective immunity against P. falciparum and P. vivax malaria, and should aid the development of effective vaccination strategies.
These articles are all part of our Combating malaria: research, prevention and treatment article collection, which is now open for submissions. The collection aims to highlight recent progress in all areas of malaria research, including vaccine development, new antimalarial agents, vector control and disease epidemiology. If you have any research you would like us to consider for inclusion in our malaria collection, please email bmcmedicineeditorial@biomedcentral.com.
 BMC Medicine: passionate about quality, transparency and clinical impact
BMC Medicine: passionate about quality, transparency and clinical impact
2013 median turnover times: initial decision three days; decision after peer review 51 days
Comments