
Knowing one’s self – self-signaling and self-fusion
It all begins with a few spores settling down on a nutritious surface. When spores wake up in close proximity to one another they start germinating at approximately the same time and grow outwards as thread-like cells (hyphae) at a similar rate. But how does a spore know that there are others and what they are up to? This creature has neither eyes nor ears, and yet it is aware that something else is around. What’s more, it knows whether that something is itself (i.e. germlings with the exact same genetic set-up) or not.
Scientists from the UK and Germany suggest 1,2,3 that germlings of the fungus Neurospora crassa sense the presence of others by a signaling molecule. Receiving the signal triggers an internal signaling cascade in the germling that leads to specific proteins localizing to the site of initial signal reception – the germling starts extending towards the other. During that process, it sends out the same signal.
Once fused, the germlings are able to exchange cellular information and organelles.
The two befriending germlings take turns in sending and receiving the signal and extending towards each other. They keep repeating this until they physically touch, which initiates a fusion process. Once fused, the germlings are able to exchange cellular information and organelles.
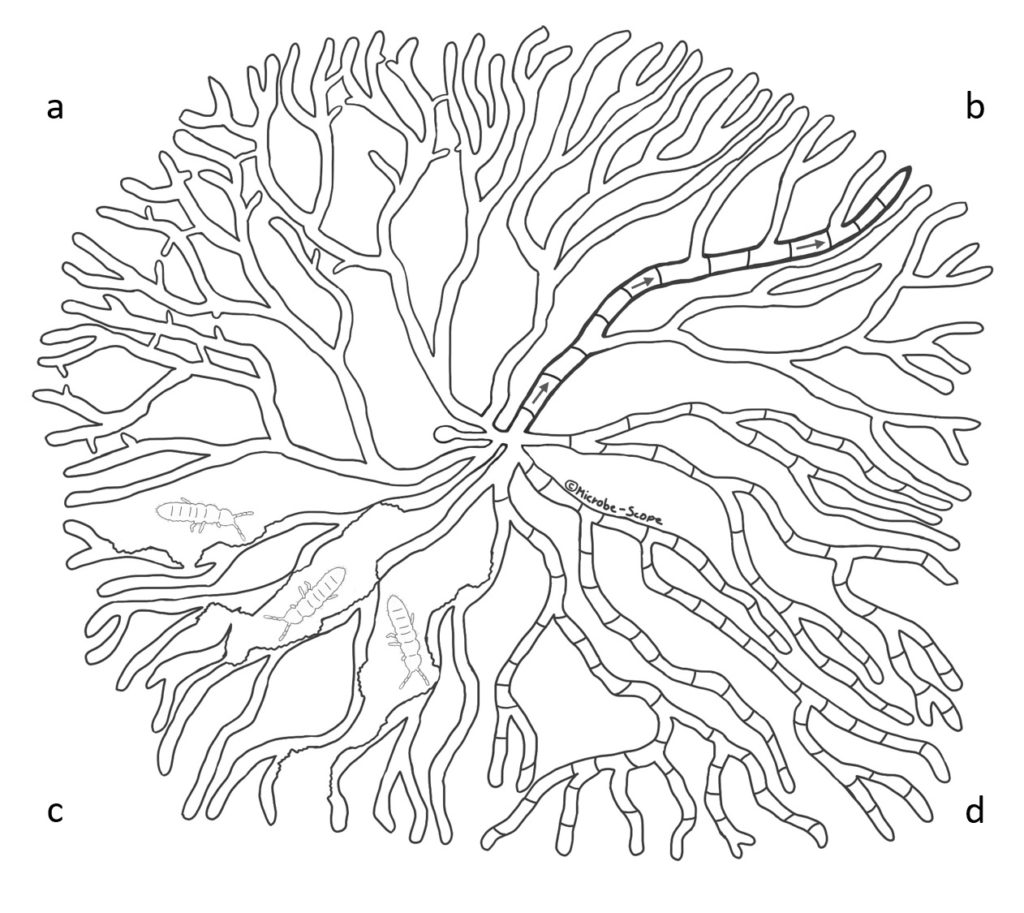
Self-recognition and fusion also work between two branches of different hyphae within the same colony allowing an even more intricate networking (Figure 2a). However, this has not been observed in all fungi. Yet, considering the orderly, sometimes almost symmetrical growth of the network of hyphae called the mycelium, it seems self-evident that the different branches keep the lines of communication open at all times.
Collectively individual – heterogeneity allows development
Despite the sometimes seemingly uniformity of a fungal colony different areas may feature different developmental structures. For example, a colony of Aspergillus nidulans tends to initiate sexual development at its center, produces asexual spores in the sub-periphery and simply extends at the periphery.
How is this possible? Studies in a fungus producing black spores, Aspergillus niger, show that heterogeneity of a colony is established and maintained by cellular barriers. Although the mycelial network is one organism, it’s not one cell – but many.
These hyphal cells are separated by cross walls called septa (Figure 2d). Septa contain pores that can be opened or closed to either allow or prevent passage of cytoplasmic content. Dutch scientists found 1,2 that in an A. niger colony the majority of these cross walls is normally closed. This mechanism allows individual hyphal cells to shuffle around their proteins, signalling molecules and internal transport pathways independently of their neighbors. However, glucose as an important nutrient is transported in specific wide hyphae even when septa are closed (Figure 2b).
Working together – the fungal colony as a dynamic and adaptive network
Scientists from the UK have taken this network research to yet another level. They feed experimental data of fungal growth into computer models to shed a light on the dynamic fluxes of signals and nutrients over time. This allows them to visualize the responses of a colony to environmental changes or predator attack.
In one of their studies, they allowed a fungus called Phanerochaete velutina to grow for a few weeks before adding springtails, natural predators to the fungus, to the colony. While the springtails munched their way through the mycelium (Figure 2c), the fungus responded to the attack by re-allocating nutrients to injured parts and rebuilding lost connections and branches. For that purpose, the colony even sacrificed unused hyphae to be able to fortify important routes. Intriguingly, the colony also prepared itself for future attacks to become more resilient.
Studies like this illustrate that a fungal colony is not just growing and getting older waiting to be eaten. It perceives and processes nutritional changes or mycelial injuries and responds to that by adaptation. To do this, it has to work as an entity. The scientists from the UK argue that the fungal colony is a network organism we have yet to fully acknowledge as such!
Comments