Schistosomes are a group of parasitic flatworms responsible for causing the neglected tropical diseases of intestinal and urinary schistosomiasis. According to the World Health Organisation, human schistosomiasis is prevalent across 78 countries, in both tropical and sub-tropical regions, and is estimated to affect over 200 million people globally. Schistosomiasis is also considered by the W.H.O. to rank second only to malaria among parasitic diseases in terms of prevalence and socioeconomic burden in an infected community.
The enormous public health and economic burden results in infected individuals and their families being stuck in a cycle of poverty; where they are unable to improve their health due to financial constraints, but also struggle to maintain economic productivity because of their poor health.
In large part, these health, economic and social consequences of schistosome infection are caused by chronic, long-term infections. Without treatment, these parasitic worms have been shown to be able to persist inside a human host for over a decade (here is a case where they had been present for over 38 years!), but how can they survive inside a host’s blood vessels for so many years?
Schistosome physiology enables long-term infection
Schistosomes have a hugely complex life cycle, with an intermediate snail host and a definitive mammalian host.
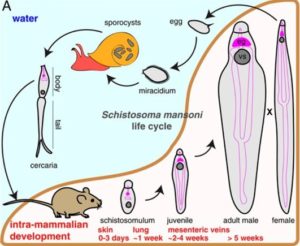
Upon mammalian host invasion, schistosomes transform through multiple body plans (Cercariae, Schistosomula and Adult), where they eventually reside in their mammalian host’s vasculature, pair with an individual of the opposite sex, and produce thousands of eggs.
However, this is not a ‘happily ever after’ for our pair of parasitic worms. The host’s vasculature is an extremely hostile environment, brimming with a range of immune components and molecules, tirelessly trying to recognise, bind and kill the parasites. To survive for such a long time inside a human host, schistosomes have (had to) become master manipulators of their environment and display evolutionarily refined characteristics to ensure their long-term survival.
It is well documented that the Schistosome outer surface layers (the tegument) are crucially important in facilitating host immune evasion and maintaining long-term intravascular infection, as this area is highly accessible to immune molecules. However, the parasites digestive tract has now been found to be equally as valuable in helping the parasite to evade the host’s immune system.
The oesophageal gland is essential for survival inside the mammalian host
Jayhun Lee and colleagues at the Morgridge Institute for Research and Howard Hughes Medical Institute of UoW- recently published invaluable research into schistosome digestive tract development, potential mechanisms behind prolonged schistosomiasis infection, and methods of host immune system evasion.
The researchers found, much to their surprise, that an accessory organ of the schistosome digestive tract called the oesophageal gland (OG) develops before the rest of the digestive system. As the digestive system hasn’t been fully formed, and blood feeding hasn’t started yet, it led Jayhun and colleagues to suggest that the OG has a role in schistosome processes beyond just nutrient uptake and host blood digestion.
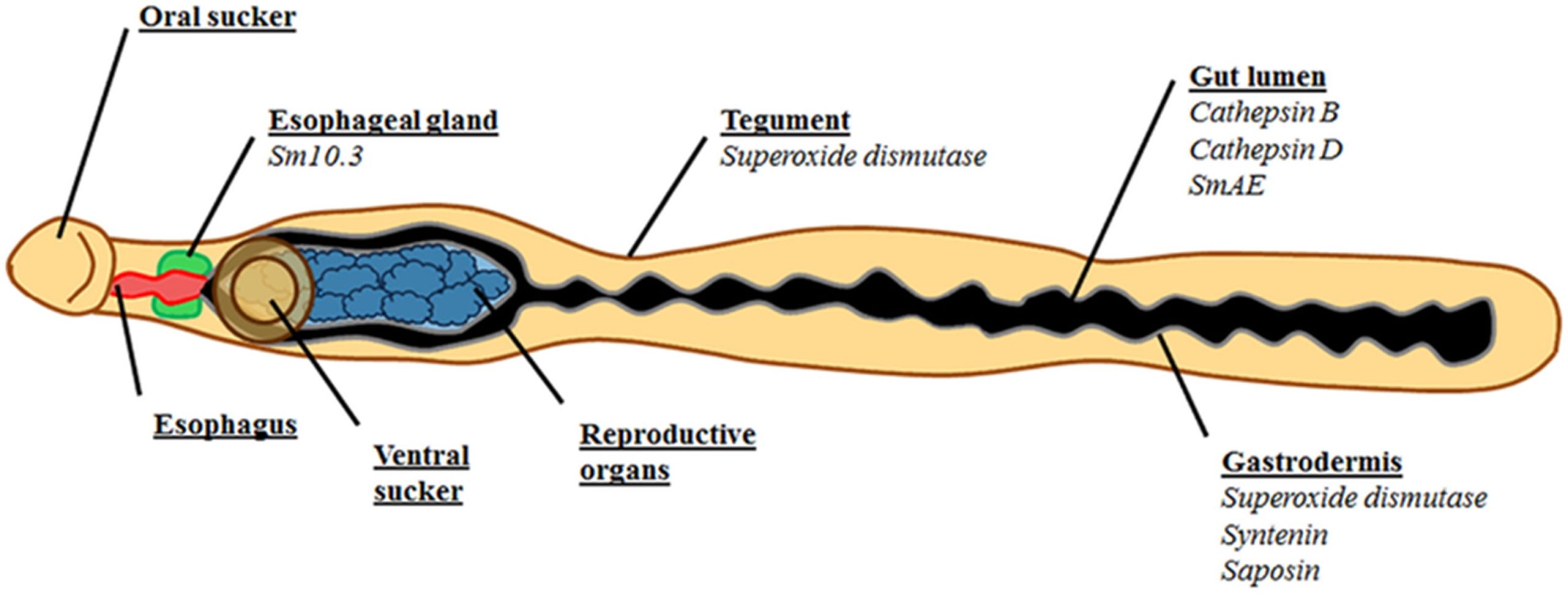
For example, the OG is known to secrete a diverse assortment of proteins and other molecules, many of which have unknown functions beyond their demonstrated host protein interaction capabilities. One hypothesis (increasingly being shown to be true) is that OG protein secretions can bind, block, and mop up host antibodies and certain other immune cells before they enter the schistosome’s blind gut, acting as a barrier to the host’s immune system. This OG secreted protein barrier could prevent immune molecules from causing direct damage to the gut, or indirect damage by further enhancing the immune response.
In one of their experiments to test the role of the OG in parasite survival, FoxA (a Forkhead-box transcription factor), a key regulator in OG development and maintenance was knocked out using RNAi, resulting in the complete absence of a normal functioning OG.
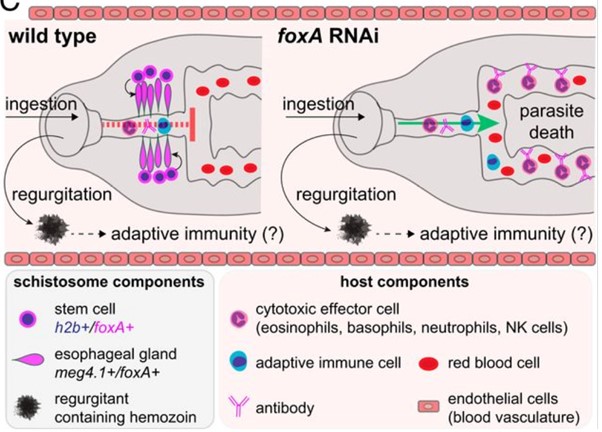
These parasites lacking an OG were rapidly killed by the host’s immune system in ‘normal’ immunocompetent mice. However, in mice genetically engineered to lack an immune response, the parasites without an OG survived- showing that, without the OG to mediate protection from the host’s immune molecules, white blood cells could gain access to the parasite’s gut, enabling recognition of parasite gut tissues, and significantly enhancing killing of the parasite from the inside out.
How can we take advantage of these mechanisms?
Identification of OG-secreted proteins (that manipulate the host’s immune system) and analysis of their interactions with host immune molecules could provide novel antigens with great potential as vaccine targets.
One such group of secreted proteins with considerable promise are the Venom Allergen-Like (VAL) proteins, a group found in a variety of other parasitic worms and organisms. Several VAL proteins have been shown to be released from the OG in Schistosoma mansoni, namely VAL-6, VAL-7 and VAL-13 proteins; however, the specific roles and exact functions of these proteins in the immune evasion process still remains largely unknown.
Additionally, the authors suggest that the identification of genes and proteins downstream of FoxA may help us to selectively disrupt the proper function of the OG, promoting and facilitating schistosome killing by the host’s immune system. Also, as the OG develops during schistosome larval stages, any therapeutic targets could be effective against both immature/larval stages and adult worms, something that the current drug of choice, Praziquantel, cannot do.
With future efforts and continued research into the OG and its associated protein secretions it is possible we may discover a highly promising, novel antigen with great promise as a vaccine target – something that will be essential to control and eliminate this disease going forward.

Comments