
When humans get infected with malaria, we have fevers, chills and will feel awful. We can openly communicate these horrible symptoms and take tests to verify the toll that malaria parasites take on our bodies. However, it is a bit more complicated when wildlife get infected with malaria.
Plasmodium parasites, the protozoans responsible for causing malaria in humans, also infect a variety of non-human primates, birds and even lizards. There is some evidence of lower malaria detection rates in chimpanzees as they get older, suggesting they are investing in protective immunity to prevent infection (and thus there should be some cost to this). Birds treated with antimalarial medication often do better, producing more healthy offspring. However, because malaria infection rarely results in an overtly sick animal, it is hard to know the real impact of these parasites on wild animals. Maybe it is not a big deal?
Researchers recently published findings of a five-year study of malaria in a natural population of mandrills (Mandrillus sphinx) in Southern Gabon. Mandrills are the world’s largest monkey and live in large groups (known as hordes) in tropical rainforests of Gabon, Cameroon, Congo and Equatorial Guinea. The research was carried out as part of the Mandrillus Project, which studies the ecology of the only habituated horde of mandrills (~220 individuals) in the world. The group is followed daily, which means there are many identifiable individuals to study over time. This longitudinal data (repeated sampling of the same individuals) is crucial for understanding disease in wild populations.
The researchers set out to understand how the environment (wet or dry season) interacts with host characteristics to determine malaria infection status and intensity of infection. They hypothesized that malaria would be more common in the wet season as the mosquitoes that transmit malaria are more common. The researchers also predicted that mandrills with infections that suppressed the immune system (simian immunodeficiency virus [SIV] and simian T-cell leukemia viruses [STLV]) were more likely to be infected with malaria. Lastly, they predicted that hosts of different ages and sexes would have different responses to malaria, and this could be detected through variation in temperature (i.e. fever) and measuring immune responses.
The unique study setting enabled the researchers to examine all their hypotheses using the same population of mandrills. Blood samples were regularly collected from a subset of individuals in the group to measure malaria infection status, co-infection with SIV and/or STLV, white blood cell profiles (neutrophil to lymphocyte ratio; an indicator of inflammation) and oxidative markers (an indicator of stress). In addition, during two sampling time points some adult mandrills were fitted with radio-collars that could record skin temperature. Combined with the daily temperature, this could help researchers determine if the animals had an elevated temperature.
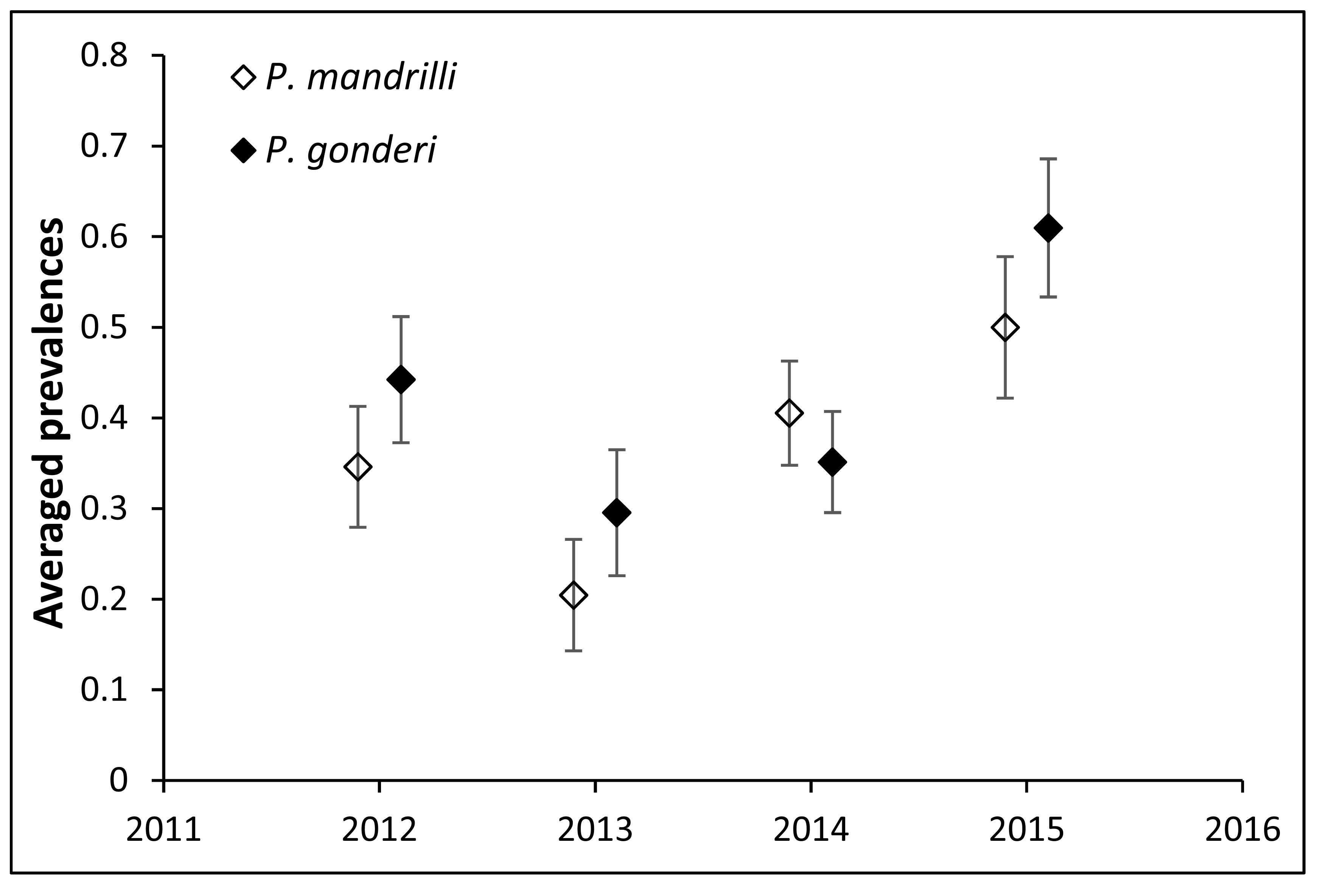
Mandrills were infected with both Plasmodium mandrilli and P. gonderi. The prevalence of each malaria parasite generally followed similar patterns between years. A greater proportion of male mandrills were infected with malaria (both species) compared to females. While P. mandrilli was detected in all ages, P. gonderi was more frequently detected in older animals (~13 years of age was the highest risk).
Infection with either SIV or STLV had various effects on malaria infections. In males over the age of 6, acute infection with SIV increased the number of P. gonderi parasites in individuals. However, chronic infection with SIV did not affect the number of parasites compared to uninfected individuals. Interestingly, while skin temperature did not influence P. mandrilli infection or abundance, high numbers of P. gonderi parasites were associated with higher skin temperatures. This suggests individuals with P. gonderi may have had a fever. P. gonderi infection was also associated with some inflammation.
The five-year study indicates there is some impact of malaria on mandrills, but the responses are complex and dependent on the parasite species. Malaria is common in this population and individuals were repeatedly infected over the observation period. There was some evidence that infection with one malaria species helped facilitate infection with the second. While this study has provided initial insight into malaria infections in a wild population, the study sample size was small. Therefore, further research is needed to better understand the complexities of these interactions.

Comments