Most parasites that are transmitted by blood-sucking insects must gain access to their vector’s mouth parts or salivary glands before they can be transmitted to a new vertebrate host during blood feeding. This often means overcoming physical and physiological barriers. This is so for trypanosomes, the parasites that cause sleeping sickness in humans. During infection in their vector, the tsetse fly, they must cross the peritrophic matrix twice and overcome immune responses before reaching the salivary glands.
The peritrophic matrix
Insects that feed on blood are stimulated to secrete a lining to their midgut when they take a meal. This lining, known as the peritrophic matrix (PM), is composed of chitin (the major component of the insect exoskeleton) and proteins. It is thought to give some protection against invasion by pathogens that may be present in the bloodmeal.
The tsetse fly PM is secreted by cells at the junction between the foregut and midgut, known as the cardia organ. This matrix forms a tube that separates the lumen of the gut from the gut epithelia. The space between the two is known as the ectoperitrophic space. The PM could act as a significant barrier to invasion by trypanosomes.
Trypanosome invasion and transmission
Two forms of trypanosome are taken up by a tsetse fly during feeding: slender forms and stumpy forms. The slender forms are lysed and shed their surface coats of glycoproteins, which are taken up by the cardia cells. These glycoproteins initially inhibit the production of a structurally robust PM. This aids the stumpy forms as, after they have transformed into a midgut-adapted form, they move through the PM into the ectoperitrophic space.
At this stage, the majority of trypanosomes are eliminated by the tsetse immune response. Very few adult flies actually become infected, although infection success is greater in newly emerged adults.
Those parasites that survive, move through the ectoperitrophic space back up to the cardia. There they must cross the PM again to enter the gut lumen of the cardia, the foregut and then the salivary glands. In many flies, infections persist in the midgut, but the trypanosomes do not invade the salivary glands. Only a small percentage of tsetse flies end up with salivary gland infections.
Events that occur in the cardia are thus crucial in determining whether the fly will transmit infection to another human, but the barriers that prevent salivary gland infections are not understood.
Events in the cardia
Aurélien Vigneron and colleagues recently reported their comprehensive investigation of molecular and cellular mechanisms in the cardia that might inhibit salivary gland invasion. Initially they demonstrated that the inability to infect the salivary glands was determined by factors in the tsetse fly rather than the parasites.

They then infected newly emerged flies and divided them into flies that only had midgut infections (inf+/-) and flies that had midgut and salivary gland infections (inf+/+) and used transcriptomics, functional genomics and microscopy to compare these groups. They looked at the expression of three PM associated genes that were downregulated in the first 3 days of infection, but not afterwards, and concluded that the initial loss of matrix integrity was not involved in the re-entry into the gut lumen.
Parasites in the cardia
Parasite density in the cardia was higher in the inf+/+ group than the inf+/- group, even though parasite density in the midgut was the same. Thus, either fewer trypanosomes were getting into the cardia in the inf+/- group, or fewer survived there.
Microscopic examination showed many parasites in the lumen of the cardia and foregut of the inf+/+ group. However, in the inf+/- group, parasites were only seen in the ectoperitrophic space of the cardia. This suggested that the PM was acting as a barrier in the inf+/- group.
PM-associated transcripts were found to be less abundant in the inf+/+ group and transcripts for two proteinaceous components were significantly reduced. The researchers confirmed the hypothesis that a compromised PM aided the movement of trypanosomes into the cardia lumen by using gene knock-down to produce flies with a compromised matrix. When subsequently infected, these group had significantly more salivary gland infections that the control groups.
Extracts from the cardia of inf+/+ flies decreased the integrity of the PM of uninfected tsetse flies, but the active components were not determined.
The authors used electron microscopy to observe parasites in inf+/+ flies massed in clusters embedded in the cardia PM in cyst-like structures and suggest this may be a “safety in numbers” strategy.
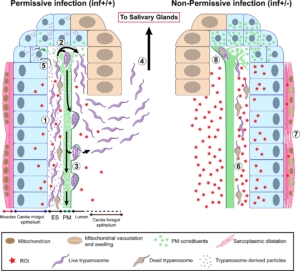
The expression of cardia genes associated with immune responses was higher in inf+/- flies and cellular damage indicative of oxidative stress was more prominent in their cardia muscle and fat-containing cells, as were levels of peroxide. In addition, trypanosomes located in the cardia ectoperitrophic space of inf+/- flies showed signs of cell death.
Conclusions
The authors conclude that salivary gland infection is inhibited in inf+/- flies by the upregulation of a strong immune response, but this results in a degree of collateral damage. In contrast, inf+/+ flies tolerate the parasites’ presence and thereby suffer less cellular damage whilst their compromised PM allows the trypanosomes to break through this barrier.
As the inf+/- strategy protects the integrity of the PM as a barrier against infection by other pathogens, it would be interesting to see whether, in the wild, the loss of cardia cellular function incurs more fitness costs and decreases survival than tolerating salivary gland infection which interferes with feeding.

Comments