The world needs a malaria vaccine.
Vaccines are the most cost effective and efficient method of protecting against infectious diseases. Smallpox was eradicated using a vaccine. This is the only human infectious disease ever eradicated. Diseases once widespread, are now close to eradication (polio, measles), because of the deployment of effective vaccines. However, malaria, one of the oldest and deadliest diseases in human history, remains without a marketed vaccine.
In 2016, WHO reported that there were 216 million cases of malaria and an estimated 445,000 deaths worldwide, the majority of cases occurring in Sub-Saharan Africa, These numbers were increased compared to 2015. Malaria experts have spent decades trying unsuccessfully to develop a vaccine against this complex, eukaryotic parasite. Out of necessity, anti-malaria programs have therefore focused on control using more conventional methods to decrease contact between infected mosquitoes and people or treating people with drugs. However, recent advances in malaria innovation have put us close to developing a malaria vaccine capable of effective protection that can be produced on a large scale.
A sporozoite-based malaria vaccine targeting infection.
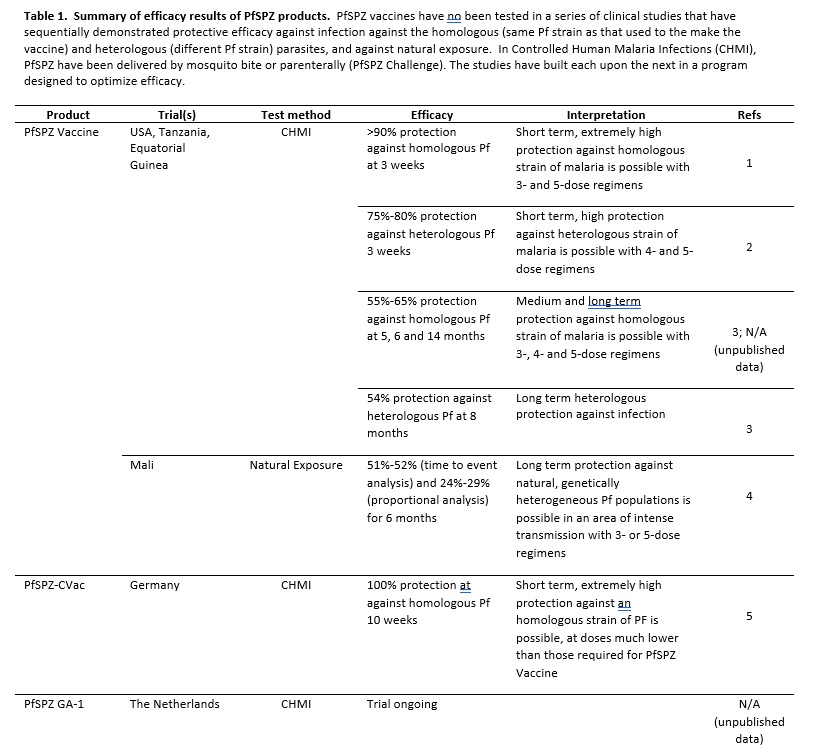
Since 2003, the biotech company Sanaria Inc. has been working to creating an effective malaria vaccine. Sanaria has developed unique technologies to manufacture vaccines, soon at phase 3 compliance, that are composed of Plasmodium falciparum (Pf) sporozoites (SPZ). These PfSPZ are attenuated either by irradiation (Sanaria® PfSPZ Vaccine) when still in the mosquito, by malaria drugs (Sanaria® PfSPZ-CVac) during liver or early blood stages, or by gene deletion (Sanaria® PfSPZ- GA1). These vaccine candidates induce powerful T cell responses in the liver that prevent infection from Pf, and thereafter the development of blood stage parasites and transmission of blood sexual stages to the mosquito. All three vaccines are being tested in human volunteers with very encouraging results summarised in the Table. Table References 1), 2), 3), 4), 5).
Malaria control in Equatorial Guinea.
An important partner in this exciting work is the Government of Equatorial Guinea (EG) which, along with three private party funders, Marathon Oil Corporation, Noble Energy and Atlantic Methanol Production Company, have committed to furthering the development of PfSPZ Vaccine to the point where it can be tested in an ambitious demonstration project for Pf elimination.
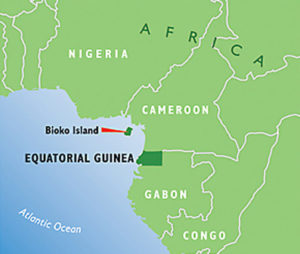
EG is a malaria endemic country in Central West Africa. With a population of around 1 million inhabitants, malaria is a fact of life for the country. In 2004, the Government and the private parties formed a public-private partnership that has spent the last 15 years controlling malaria on Bioko Island and parts of the mainland in an effort to reduce malaria’s burden on the population.
The Bioko Island Malaria Control Project (BIMCP) implemented by the U.S. NGO, Medical Care Development International (MCDI) and the Ministry of Health and Social Welfare of the Government of EG, has reduced malaria prevalence from 74% (by thick blood smear) in 2003 to 11% (by rapid diagnostic test) in 2017 in 2-14-year olds, infant mortality due to malaria infection by 85% (by rapid diagnostic test), and anemia by similar numbers. However, it has proven difficult to eliminate malaria from the region despite the increased intensity of malaria interventions. MCDI and its funders, recognizing the challenges being faced in such an endemic environment decided in 2013 to partner with Sanaria, the Ifakara Health Institute (IHI, Tanzania), and the Swiss Tropic and Public Health Institute (Swiss TPH) to form the Equato-Guinean Malaria Vaccine Initiative (EGMVI).
Building the capacity for global health research through vaccine development.
The aim of the EGMVI is to conduct a series of clinical trials that will advance PfSPZ Vaccine through to phase 3 clinical trials and eventually test the public health utility of the vaccine in malaria elimination projects. To do this, the EGMVI is training a cohort of national clinicians and nurses, laboratory technicians, data managers, and community managers, who will eventually form the nucleus of the first EG public health institute.
The clinical trials are testing PfSPZ Vaccine and PfSPZ-CVac for safety and efficacy in EG. The EGMVI is unique in that the project has managed to conduct the country’s first human subject clinical trials using an investigational product according to international standards of good clinical practice and safety, as well as put the first Equatoguinean researchers on the map in terms of vaccine research. For instance, in April 2018, Equatoguinean EGMVI researchers, along with their Equatoguinean colleagues in the BIMCP, will present 15 papers and posters at the Multilateral Initiative on Malaria meeting in Dakar, Senegal.
Also unique to the EGMVI is the South-South collaboration that has been established between EG and Tanzania. The collaboration has resulted in extensive training conducted at IHI’s center in Bagamoyo, Tanzania, to bring Equatoguinean staff up to speed regarding conduct of clinical trials using human subjects. IHI staff have been instrumental in running the first two trials in EG. This work has included acting as the principal investigator, coordinating the clinical trial and running the laboratory required to process clinical samples and trial data. Meetings between the EG and Tanzanian Ministries of Health have explored future collaborations between the two countries.
Lastly, the EGMVI opportunity has opened other doors for the EG Ministry of Health and Social Welfare, whereby EG has been invited to participate in the African Vaccine Regulatory Forum and is a sitting member of the International PfSPZ Consortium. Future efforts will be sought to have EG participate in pan African initiatives through WHO – AFRO and UNICEF.
To date EGMVI has tested PfSPZ Vaccine or PfSPZ-CVac in 168 volunteers aged 6 months to 65 years. Safety and tolerability have been excellent and more importantly, the communities from which the participants have been recruited have become important partners in the EGMVI and EG’s efforts to develop a solution to eliminate malaria from the country. With the safety trials finalized, the next step is to conduct a trial to optimize the dosage regimen of PfSPZ Vaccine. This will be followed in 2019 by a Phase 3 study designed to assess PfSPZ Vaccine’s safety, tolerability, and protective efficacy in the endemic malaria setting of Bioko Island.
An integrated approach to eliminate malaria from Bioko Island.
The EGMVI is targeted to be the first location in sub-Saharan Africa to eliminate malaria using a vaccine in addition to standard malaria control efforts. The long-term plans are to combine standard malaria control – insecticide treated bednets, indoor residual spraying, larval control, diagnosis and treatment, and mass drug administration – with high coverage of PfSPZ Vaccine in the whole population to halt transmission and eliminate malaria, initially from foci in a cluster randomized study, and eventually from the whole of Bioko.
We look forward to sharing that story as it unfolds over the next few years.

Nice article Tom, and great work too!
Thanks