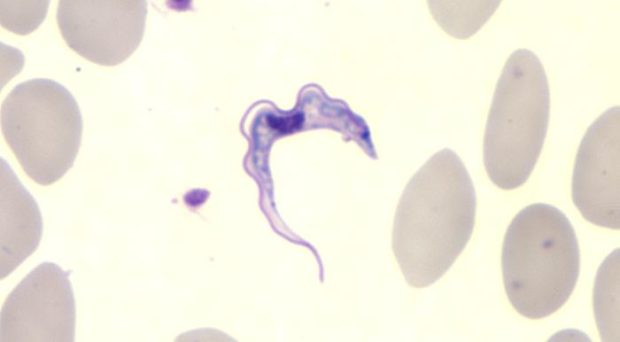
A parasite that infects your blood and brain with the ability to alter your sleep pattern, cause confusion and personality changes, eventually leading to coma and death might sound like something from a film, but trypanosomes are parasites that can do just that. In parts of Sub-Saharan Africa, Sleeping Sickness, or Human African Trypanosomiasis, is a disease that is caused when an infected tsetse fly bites a human and injects trypanosomes as it takes a blood meal.
Although human cases are currently at a record low due to intensive control and surveillance programmes, epidemics in the past have had catastrophic death tolls in affected communities. Depending on the parasite species, patients can suffer symptoms for months or years before the extreme neurological symptoms that give the disease its name, and lead, if untreated, to death. Some species of trypanosome infect animals (Animal African Trypanosomiasis), resulting in wasting and death of domestic animals including cattle and horses. Also known as nagana, this disease has wide-ranging impacts on socio-economic development in affected regions, including limitation of food supply (meat, milk) and the use of animals in plot cultivation or transport and loadbearing.
The history of African Trypanosomiasis

It was in the late 1800s that the parasite was first identified by Sergeant Major Major David Bruce, and experiments began to understand the disease in both animals and humans. It is an fortunate by-product of the slave trade that much interest was generated at this time from the presence of illness in Africans transported to Western countries.
While it had been known that some trypanosomes do infiltrate other tissues and lymph nodes, it was understood that the parasites are adapted to survive and multiply within the blood system for transmission to the insect vector.
However, as early as 1895, Bruce observed that an animal could transmit the disease despite having no detectable parasites in the blood, stating “In what part of the organism these parasites exist during their absence from the blood is a curious point”. This has been a curious point for researchers ever since, although it seemed probable that these patients likely had trypanosomes circulating at such low numbers that they couldn’t be seen under the microscope.
Where do Trypanosomes hide?
Over 100 years later, at least a part of this mystery is understood – a combination of three papers published in the last year have shed new light on the mysterious life cycle in the host. These developments have revealed that parasites have the ability to adapt and survive under the skin and within fat tissues.
Last year a team of researchers led by Anette Macleod reported that, in mouse infections, populations of parasites can be found under the skin, and can be transmitted to a biting tsetse even in the absence of parasites in the blood. A second paper, by Guy Caljon and colleagues, used fluorescent-tagged parasites to show that parasites are deposited into the skin by the tsetse bite, and are able to multiply in the dermis. Again, this study showed that the skin-dwelling populations could be ingested by feeding tsetse flies.
Finally, a paper co-authored by Sandra Trindade and Filipa Rijo-Ferriera described how their team investigated adipose (fat) tissues, identifying populations of parasites that have specific adaptations to this environment, including a type of metabolism not found in bloodstream parasites (see also a previous BugBitten blog). It is as yet uncertain if these parasites, named Adipose Tissue Forms (ATF), can be transmitted, and may give separate advantages such as protection from the host immune response, a more suitable environment for growth or a reservoir from which to repopulate the bloodstream as the immune responses clear parasites.
All three papers here have used an animal model of Trypanosoma brucei brucei
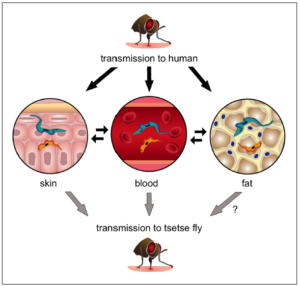
infected mice, which, although closely mimicking human infection, is an unnatural host-parasite combination. Further work will be needed to validate these findings in natural infections. The Macleod group reviewed historical skin samples collected from an independent screening programme and found trypanosome-like organisms in a small number of patients, although they could not confirm they were definitively species that cause Sleeping Sickness.
Further work is now needed to understand how our current drug treatments affect parasites that are not in the blood stream – are trypanosomes able to “hide” in the skin or adipose tissues? Can we still accurately diagnose patients and animals as infected, based solely on blood tests? What proportion of hosts are infected but have no detectable blood parasites? How do these hosts contribute to transmission in the field?
As so often, new discoveries seem to raise as many questions as they answer but perhaps these findings go some way to answering David Bruce’s musings from a century ago.

Comments