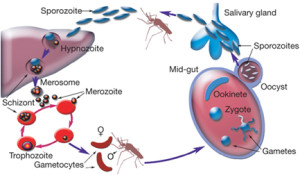
Plasmodium, the parasite that causes malaria, has a famously complicated lifecycle. It is transmitted between humans only via mosquito vectors, when specially differentiated ‘gametocytes’ are taken up in a mosquito’s blood meal and undergo sexual reproduction inside its gut. Parasites then migrate to the salivary glands and are eventually injected into another human in a subsequent bite.
Clearly, the mosquito is far from being a mere ‘flying syringe’; rather it is an integral part of the parasite’s lifecycle, hosting a series of unique differentiated forms and the only sexual stage of the entire process.
So, besides the opportunity for a genetic reshuffle during meiosis, what other changes might the parasite experience as it passes through a mosquito? One intriguing possibility is an epigenetic reset: a wiping-clean of the epigenetic memory that is key to controlling antigenic variation, immune evasion and chronicity in this parasite. This idea was explored in a recent Opinion piece published in Plos Pathogens by Philip Spence, who recently conducted an intriguing study in this area.
Virulence after the mosquito
In 2013, Spence and colleagues reported in Nature that passage through mosquitoes attenuated parasite virulence in the mouse malaria model P. chabaudi. Mice infected with P. chabaudi by serial blood passage (direct injection from mouse to mouse, bypassing the natural route) suffered from severe malaria, whereas mice infected with parasites via mosquitoes developed a lower parasitaemia and a mild, chronic disease.
These results paralleled observations from the early 1900s, when human malaria, caused by P. falciparum or P. vivax, was used to induce fevers as a therapy for syphilis. Here too, the parasites seemed to grow more virulent if not regularly passed through mosquitoes. But what was the biological mechanism? What could be happening to the parasites inside the mosquito to attenuate their virulence? Spence et al. have suggested a complete re-setting of a major family of virulence genes called pir.
Virulence genes reset
Pir genes code for variant surface antigens (VSA) that are expressed on parasite-infected erythrocytes. There are hundreds of pirs in most Plasmodium species and they are thought to facilitate immune evasion by regularly switching to keep the parasites one step ahead of adaptive immunity. RNA sequencing revealed a wide repertoire of pirs (called ‘cirs’ in P. chabaudi) expressed in populations that originated via mosquito bites. By contrast, the parasites transmitted by blood passage had narrowed down to express just a few dominant genes, which were not reset when they entered a new mouse.
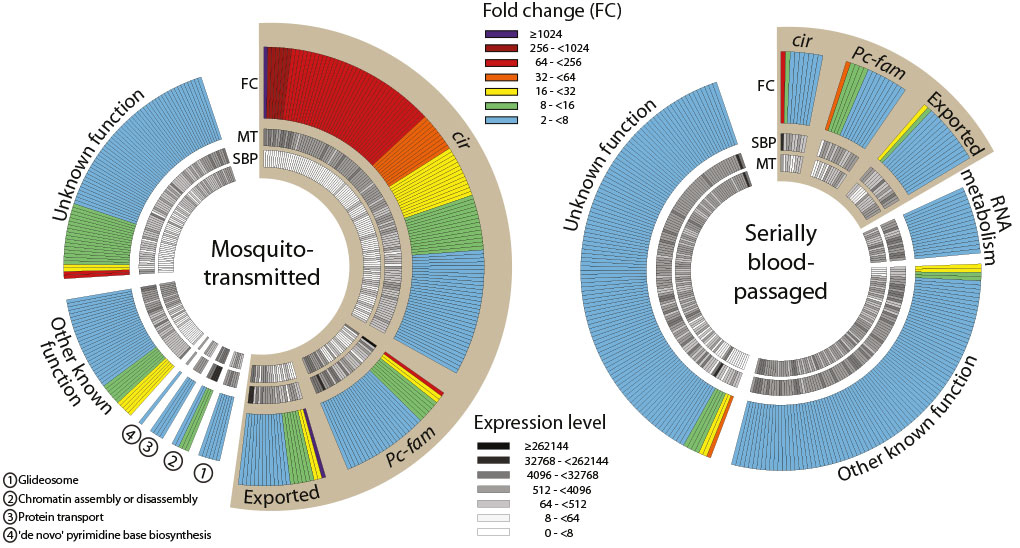
It is not known whether these populations were composed of individual parasites each expressing a single cir gene, or of parasites expressing a more or less diverse repertoire of many cirs all at once.
These striking data appeared to some researchers to be counterintuitive. If VSA have evolved to facilitate immune evasion, why would a parasite reveal its entire repertoire by exposing it, even at a low level, to immune recognition upon entering a new host? Wouldn’t it be more advantageous to express one or a few antigens sequentially, keeping most of them in reserve to facilitate a long chronic infection? Furthermore, shouldn’t the immune system respond more easily to the restricted antigen set of the blood-passaged parasites, and thus control the infection more, rather than less, efficiently?
The authors’ counter-argument is that mosquito infection elicits a more ‘appropriate’ immune response, which controls parasitaemia without the excessive immunopathology caused by blood-passaged parasites. It remains unclear whether this is primarily down to the VSA repertoire, or to something else, such as immune priming in the stages before the erythrocytes that are bypassed by blood-passage of infected erythrocytes with no liver stages necessary.
High-profile, counterintuitive results are often controversial, and some researchers have disputed the generality of mosquito attenuation, citing other strains of P. chabaudi, handled slightly differently, that did not show such marked attenuation. Others have suggested alternative explanations for the attenuated virulence, not based on the antigenic repertoire.
Could it be, for example, that serial blood passage tends to evolve highly virulent but poorly transmissible genotypes that simply get ‘bottlenecked out’ in the mosquito? In short, although the altered gene expression profile is tantalising, it may not be definitively ‘virulent’.
In the field rather than the lab
These are important questions, but so too is the question of how this might inform our understanding of human malaria. In the real world (leaving aside a few historic syphilis patients) humans always contract malaria via a mosquito bite. Human disease is primarily caused by either P. vivax, which has a large family of pir genes analogous to P. chabaudi, or by P. falciparum, which has both pirs and also a unique family of variantly-expressed genes called vars. These encode a dominant surface antigen and virulence factor called P. falciparum Erythrocyte Membrane Protein 1 (PfEMP1). The epigenetic basis of variant expression amongst var genes is much better characterised than for any pir family… so can mosquitoes ‘wipe the epigenetic slate’ and reset the var expression profile too? This is a question of real medical importance, for a particular set of PfEMP1s, highly expressed in early infections and in malaria-naïve hosts, are well-established to associate with severe and lethal disease.
Rare volunteer studies in which humans were infected with P .falciparum via mosquito bites do offer indirect evidence for such re-setting, because many different var transcripts were detected in the arising populations. (Again, whether this represents fast and diverse switching, or multiple genes expressed in single parasites, remains unknown. The prevalent dogma is that var antigenic variation is enforced at one-gene-per-parasite, yet parasites cultured ex vivo can express anything from a single gene to half a dozen.) And would these infections, if left untreated, have proved less virulent than equivalent blood-passaged infections?
Further work on this phenomenon is clearly required. At the level of molecular biology, characterising the epigenetic profile of parasites at each mosquito stage will be a priority – and a challenge, given the technical difficulty of accessing these stages in large, pure quantities. At the level of host pathology, meanwhile, it is equally difficult to study the evolution of untreated human infections. However, near-elimination situations could prove an unexpected gift, because the same parasite genotype can be passed around clonally when malaria is very sparse, as recently reported by Bei et al. in Senegal. Here, the same parasite expressed similar or identical var genes in multiple hosts, providing perhaps the strongest evidence to date that epigenetic re-setting in the mosquito does not entirely wipe the slate after all. As usual in malaria biology, the story is seldom simple!

Comments