
Lifestyle changes including increased consumption of high fat diets have caused a considerable rise in individuals who are overweight and obese in the past decades. Obesity is a major risk factor for type 2 diabetes. These individuals have increased blood sugar (hyperglycemia) and cholesterol levels, leading to various other health complications. According to International Diabetes Federation (IDF), 1 in 11 adults have diabetes, and 21% of the world’s population will be affected by diabetes by 2050.
Several clinical trials have proposed the useful effects of lifestyle intervention for controlling obesity and diabetes, however, in many cases restoring the glycemic index to normal levels does not ameliorate diabetic complications. This has been attributed to “metabolic memory” or “legacy effect”. High glucose conditions induce long-term cellular changes which are not completely restored after normalizing glycaemia. Metabolic memory due to programing has been attributed to induced epigenetic modifications like DNA methylation which alter normal gene expression and cell function.
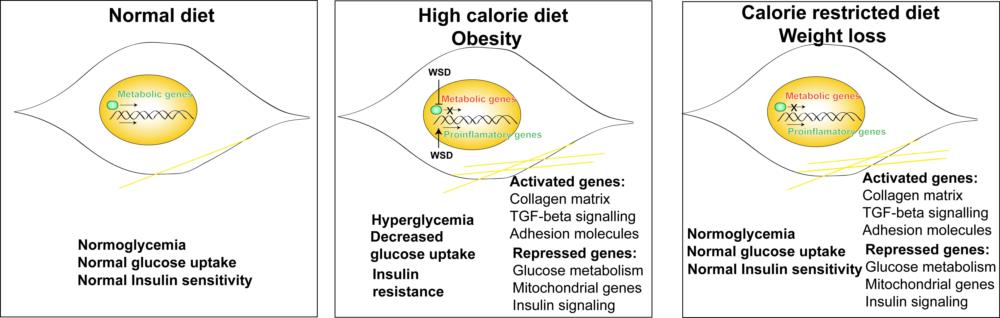
Our study, published recently in BMC Genomics, assessed the physiological and cellular effects of high caloric diet induced hyperglycemia followed by caloric restriction, a common approach of controlling obesity in accordance with metabolic memory in non-human primates. Skeletal muscle being the major site of glucose intake, was the tissue of interest for this study. We included 6 male rhesus macaques and put them on western style calorie dense diets for 6 months followed by a calorie restricted diet to 70% of their required caloric intake.
We observed that a high-fat diet increased body weight, blood glucose and caused insulin resistance in macaques and calorie restriction in the same animals returned these levels to normal but had only a partial effect on fat loss. Findings from our transcriptional analysis showed that high-fat diet reprograms the skeletal muscle to induce changes which persist even after calorie restriction. Genes involved in glucose metabolism and insulin signaling remained downregulated post high-fat diet and caloric restriction while those involved in extracellular matrix remodeling remained upregulated. We also suspect that changes in the inflammatory response induced by high fat diet might induce ROS-related stress and other metabolic pathway changes which adapts the skeletal muscle to high fat environment. Related to metabolic memory, we identified a major epigenetic modifier SETD7 to be expressed at reduced levels following calorie restriction.
To improve long term problems associated with obesity it is important to identify the changes it induces within the cell. Identification of these factors through our study as compared to other studies provides possible cellular changes within the cell which can be targeted through medical intervention in addition to lifestyle intervention to improve health conditions.
Comments